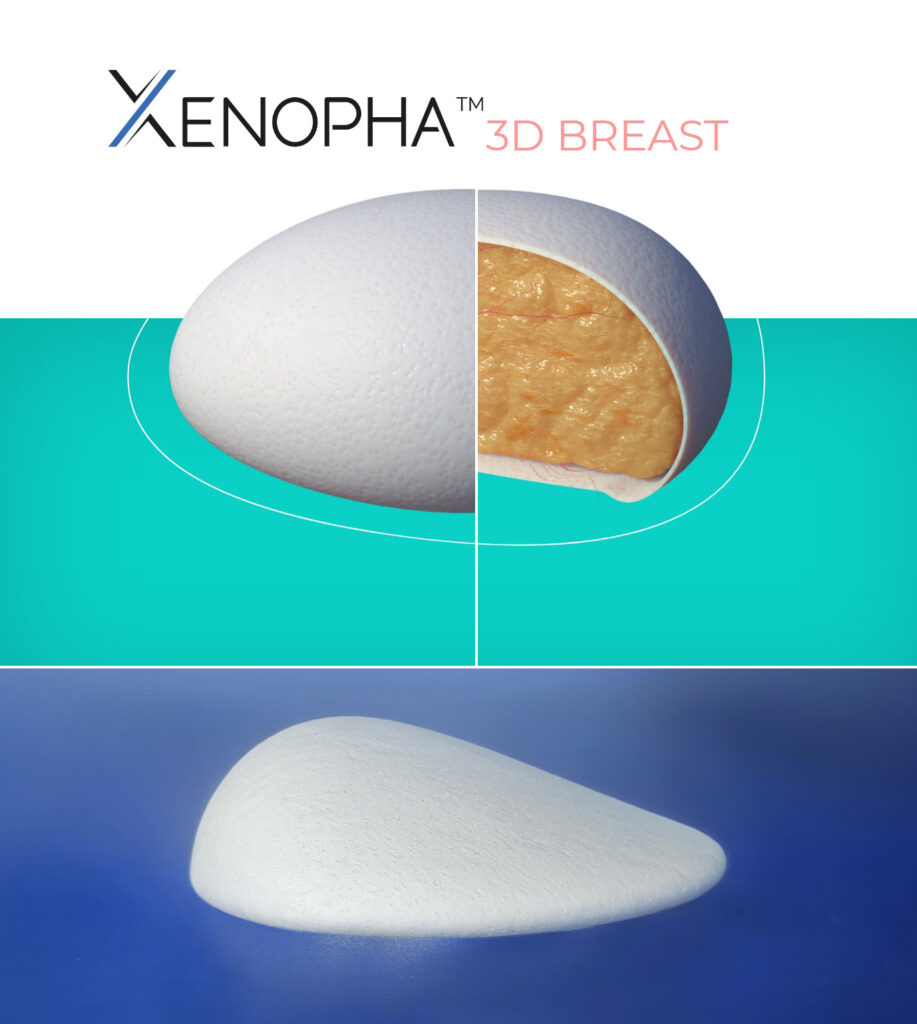
The combination of the know-how of Apha Biomat and Meccellis Biotech:
Innovative and bringing hope
to patients around the world : XENOPHA
The P4HB is produced by natural fermentation
The P4HB is a PHA (polyhydroalkanoate) biopolymer. It is recognized for its biocompatible properties. It is made up of monomers naturally present in the human body. Once implanted, P4HB is degraded by hydrolysis.

Reduce our impact on the environment
The use of biopolymers in the medical field is one of the solutions to meet the environmental and health challenges we face. By using biodegradable and non-toxic materials, we can reduce our impact on the environment while improving the health and well-being of patients and we are proud to contribute to this. We are convinced that biopolymers have a crucial role to play in the future of medicine and we are delighted to be part of this sustainable medical revolution.

French manufacturer of medical grade biopolymer (P4HB)
Team

Fabien Dolbeau
COO

Anthony PéRèS
CTO

Guillaume HOFMANSKI
Director of clinical relations

Emilie ALAZARD PéRèS
Industrial manager

Claire CISTERNI
Clinical affairs manager

Sophie RENÉ
Quality and Regulatory Affairs Manager

Morgane COUSSINE
Production operator

Cassandra POUVREAU
Production operator

Béatrice KONIECZNY
Accountant secretary
Sponsors
News

75 rue de Québec 17000 La Rochelle - France
+33 (0) 5 17 81 07 63
Contact
Are you interested in what we do at Apha Biomat? Are you curious? We're here to explain everything to you!